At-home testing for type 1 diabetes could offer additional method of screening young people for the condition
Researchers to assess if at-home tests for young people could represent a “major breakthrough”.
Researchers have begun tests to look at the effectiveness of at-home tests for young people who could be at risk of developing type 1 diabetes.
A new at-home test to diagnose type 1 diabetes is being investigated in a major UK study led by researchers at the National Institute for Health and Care Research (NIHR) Oxford Biomedical Research Centre.
The study will invite 90 children and young people across two groups to assess the test and determine whether at-home type 1 diabetes testing could be used routinely across the NHS.
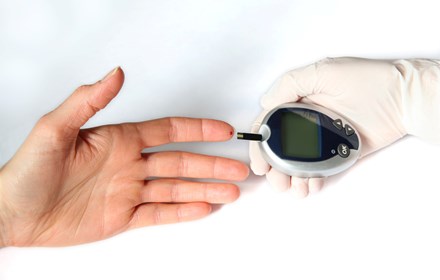
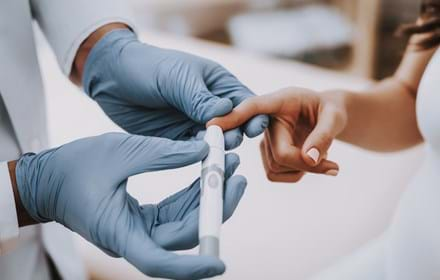
The study will assess the GTT@home test, developed by Digostics, a UK-based digital clinical diagnostics and diabetes home testing provider, for use with children.
GTT@home is an Oral Glucose Tolerance Test (OGTT) that uses finger-prick blood samples to measure how well the body processes glucose and has been shown to be as accurate as hospital-based OGTTs when used with adults.
The GTT@home test has already been implemented at NHS trusts in South East England, with more than 2,500 women screened for gestational diabetes in pregnancy since its recent launch. This will be the first study in children.
Study lead Rabbi Swaby, Clinical Research Fellow in the Nuffield Department of Medicine, said: “Early diagnosis of type 1 diabetes relies on OGTTs, but we know these tests are not well tolerated, especially by very young children.
“At-home testing could be a major breakthrough for reducing the burden of testing. We look forward to seeing the results of our study to help us understand how GTT@home might be used in routine care.”
Type 1 diabetes is the most common childhood autoimmune disease, affecting around one in 350 children. It is caused by the destruction of the insulin-producing cells in the pancreas, resulting in elevated blood glucose levels. It affects every aspect of health and wellbeing, resulting in life-long insulin dependence and an increased risk of major health problems such as heart disease, blindness, and kidney failure.

The development of type 1 diabetes is now well understood and comprises 4 stages.
- Stage 1 is the start of type 1 diabetes, when the presence of autoantibodies confirms the autoimmune destruction of the cells that produce insulin.
- In Stage 2, reduced insulin production leads to elevated blood glucose levels.
- In Stage 3 an individual has the clinical diagnosis of type 1 diabetes and symptoms may begin to appear due to significant loss of insulin-producing cells.
- Stage 4 is the long-term stage of type 1 diabetes, requiring consistent insulin management to control blood glucose levels.
Manufacturers said OGTT is the “gold-standard” test recommended for defining which stage a person with type 1 diabetes is in and is the most sensitive test for monitoring for the early onset of Stage 3. Hospital-based OGTTs are often not well tolerated because they require a drip inserted into the vein, and several blood samples taken over 2-3 hours in a healthcare setting.
Type 1 diabetes is estimated to cost the UK more than £1.8 billion every year. In around 40% of children the diagnosis of type 1 diabetes is not made until the child has become severely unwell and in “diabetic ketoacidosis” (DKA), a life-threatening diabetes complication.
Researchers said an effective type 1 diabetes screening programme would help to diagnose the condition in children before they enter DKA.
The GTT@home test kit contains the test device, finger prickers for blood sample collection, a preformulated glucose drink, and detailed instructions.
The process begins with an initial finger prick blood sample, followed by the consumption of the glucose drink. Two hours later, a second blood sample is taken.
With standard hospital-based OGTTs, children would have had to fast overnight, travel to a clinic early in the morning and then undergo the two blood draws two hours apart while remaining fasted.
The GTT@home device can instantly analyse both blood samples and shares accurate and timely results directly with the study teams.
Immediate analysis of samples eliminates the risk of sample degradation that can affect hospital-based OGTTs when samples are not sent to the lab immediately, improving diagnostic reliability.
James Jackson, CEO and Founder of Digostics, said: "We are very pleased to be partnering with the NIHR Oxford Biomedical Research Centre on this study.
"We believe that there is huge potential for the widespread use of at-home OGTT testing to diagnose type 1 diabetes. Not only is it more attractive for young children to be able to be tested at home, but the results from our GTT@home test are not affected by the time-dependent sample degradation that can lead to false negative OGTT results with in-clinic testing."
Dr Rachel Besser, who leads the T1Early programme at the University of Oxford and is overseeing this study, said: “A more practical and reliable alternative to the in-clinic OGTT would be a welcome advance and could improve the experience and therefore the uptake of children who have an OGTT as part of their care.”
I would like to make a regular donation of
I would like to make a single donation of
There are lots of ways to raise money to support
people living with all forms of diabetes.
Bake, Swim, Cycle, Fly ... Do It For DRWF!
Fundraise with us
Recent News